Functional Groups | Biology for Majors I
Mục Lục
Learning Outcomes
- Identify the attributes of molecules with hydroxyl groups
- Identify the attributes of molecules with carboxyl groups
- Identify the attributes of molecules with amino groups
- Identify the attributes of molecules with phosphate groups
- Identify the attributes of molecules with methyl groups
- Identify the attributes of molecules with carbonyl groups
- Identify the attributes of molecules with sulfhydryl groups
Functional groups are groups of atoms that occur within organic molecules and confer specific chemical properties to those molecules. When functional groups are shown, the organic molecule is sometimes denoted as “R.” For example, ethanol is typically drawn like this:

In order to condense the structure and focus on the hydroxyl group (the oxygen and hydrogen bound to the second carbon), everything besides the hydroxyl group would replaced with an R, as follows:

Note: R doesn’t always stand for the same organic molecule. It can stand in for an infinite variety of molecules.
Functional groups are found along the “carbon backbone” of macromolecules which is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Molecules with other elements in their carbon backbone are substituted hydrocarbons. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
Properties of Functional Groups
A functional group can participate in specific chemical reactions. Some of the important functional groups in biological molecules include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
Classifying Functional Groups
Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity. An example of a hydrophobic group is the non-polar methane molecule. Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acid heads that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H+) from the —COOH group resulting in the negatively charged —COO– group; this contributes to the hydrophilic nature of whatever molecule it is found on. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
Table 1. Important Functional Groups in Biology
Functional Group
Structure
Properties
General Features
Hydroxyl

Polar
Hydrophilic
Characterized by presence of H and O
Simple structure
Sulfhydryl

Polar
Characterized by presence of S
Simple branched structure
Methyl
![]()
Nonpolar
Characterized by presence of H and C
Simple structure
Carbonyl

Polar
Characterized by central C and O
Bound to 2 organic side groups
Double bond to oxygen increases the polarity
Carboxyl

Charged, ionized to release H+. Since carboxyl groups can release H+ ions into a solution, they are considered acidic.
Characterized by central C bound to O and OH
Acidic
Amino

Charged, accepts H+ to form NH3+. Since amino groups can remove H+ from solution, they are considered basic.
Characterized by presence of N
Branched structure
Phosphate

Charged, ionizes to release H+. Since phosphate groups can release H+ ions into solution, they are considered acidic.
Acidic
Characterized by presence of P
Complex structure
Practice Questions
Fructose is a common sugar that you’ve probably come into contact with in your life. What functional groups can be found in a fructose molecule?

Show Answer

Fructose has hydroxyl and carbonyl groups. The hydroxyl groups are circled in red, and the carbonyl group is circled in purple:
Leucine is an amino acid that plays an important role in muscle development. What functional groups can be found in a leucine molecule?

Show Answer

Leucine has carboxyl, amino, and methyl groups. The carboxyl group is circled in blue, the amnio group is circled in green, and the methyl group is circled in purple.
Hydrogen Bonds between Functional Groups
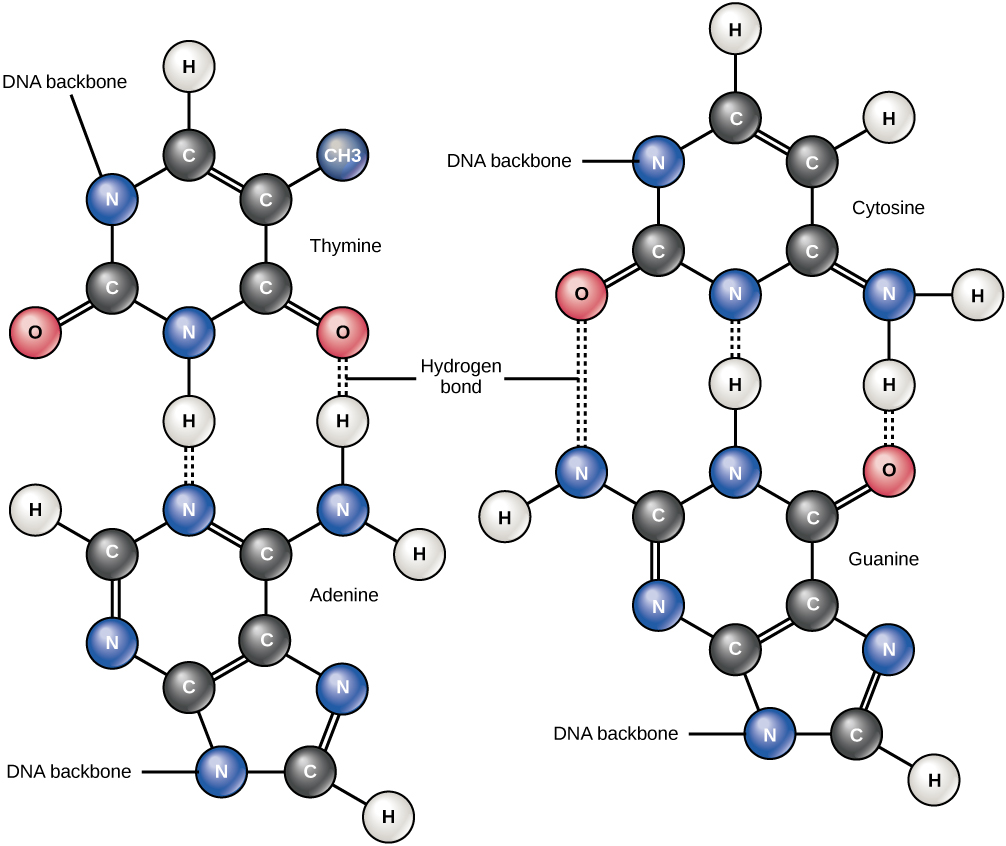
Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate, as illustrated in Figure 1.
In Summary: Functional Groups
The unique properties of carbon make it a central part of biological molecules. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon has four electrons in its outermost shell and can form four bonds. Carbon and hydrogen can form hydrocarbon chains or rings. Functional groups are groups of atoms that confer specific properties to hydrocarbon (or substituted hydrocarbon) chains or rings that define their overall chemical characteristics and function.
Try It
Contribute!
Did you have an idea for improving this content? We’d love your input.
Improve this pageLearn More






