Hydroxyl Group Substitution
Nucleophilic Substitution of the Hydroxyl Group
The chemical behavior of alkyl halides can be used as a reference in discovering analogous substitution and elimination reactions of alcohols. The chief difference, of course, is a change in the leaving anion from halide to hydroxide. Because oxygen is slightly more electronegative than chlorine (3.5 vs. 2.8 on the Pauling scale), the C-O bond is expected to be more polar than a C-Cl bond. Furthermore, an independent measure of the electrophilic characteristics of carbon atoms from their NMR chemical shifts (both 13C and alpha protons) indicates that oxygen and chlorine substituents exert a similar electron-withdrawing influence when bonded to sp3 hybridized carbon atoms. Despite this promising background evidence, alcohols do not undergo the same SN2 reactions commonly observed with alkyl halides. For example, the rapid SN2 reaction of 1-bromobutane with sodium cyanide, shown below, has no parallel when 1-butanol is treated with sodium cyanide. In fact, ethyl alcohol is often used as a solvent for alkyl halide substitution reactions such as this.
CH3CH2CH2CH2–Br + Na(+) CN(–)  CH3CH2CH2CH2–CN + Na(+) Br(–)
CH3CH2CH2CH2–CN + Na(+) Br(–)
CH3CH2CH2CH2–OH + Na(+) CN(–) 
The key factor here is the stability of the leaving anion (bromide vs. hydroxide). HBr is a much stronger acid than water (by more than 18 orders of magnitude), and this difference is reflected in reactions that generate their respective conjugate bases. The weaker base, bromide, is more stable, and its release in a substitution or elimination reaction is much more favorable than that of hydroxide ion, a stronger and less stable base.
A clear step toward improving the reactivity of alcohols in SN2 reactions would be to modify the –OH functional group in a way that improves its stability as a leaving anion. One such modification is to conduct the substitution reaction in a strong acid, converting –OH to –OH2(+). Because the hydronium ion (H3O(+)) is a much stronger acid than water, its conjugate base (H2O) is a better leaving group than hydroxide ion. The only problem with this strategy is that many nucleophiles, including cyanide, are deactivated by protonation in strong acids, effectively removing the nucleophilic co-reactant required for the substitution. The strong acids HCl, HBr and HI are not subject to this difficulty because their conjugate bases are good nucleophiles and are even weaker bases than alcohols. The following equations illustrate some substitution reactions of alcohols that may be affected by these acids. As with alkyl halides, the nucleophilic substitution of 1º-alcohols proceeds by an SN2 mechanism, whereas 3º-alcohols react by an SN1 mechanism. Reactions of 2º-alcohols may occur by both mechanisms and often produce some rearranged products. The numbers in parentheses next to the mineral acid formulas represent the weight percentage of a concentrated aqueous solution, the form in which these acids are normally used.
CH3CH2CH2CH2–OH + HBr (48%)  CH3CH2CH2CH2–OH2(+) Br(–)
CH3CH2CH2CH2–OH2(+) Br(–)
 CH3CH2CH2CH2–Br + H2O SN2
CH3CH2CH2CH2–Br + H2O SN2
(CH3)3C–OH + HCl (37%)  (CH3)3C–OH2(+) Cl(–)
(CH3)3C–OH2(+) Cl(–)  (CH3)3C(+) Cl(–) + H2O
(CH3)3C(+) Cl(–) + H2O  (CH3)3C–Cl + H2O SN1
(CH3)3C–Cl + H2O SN1
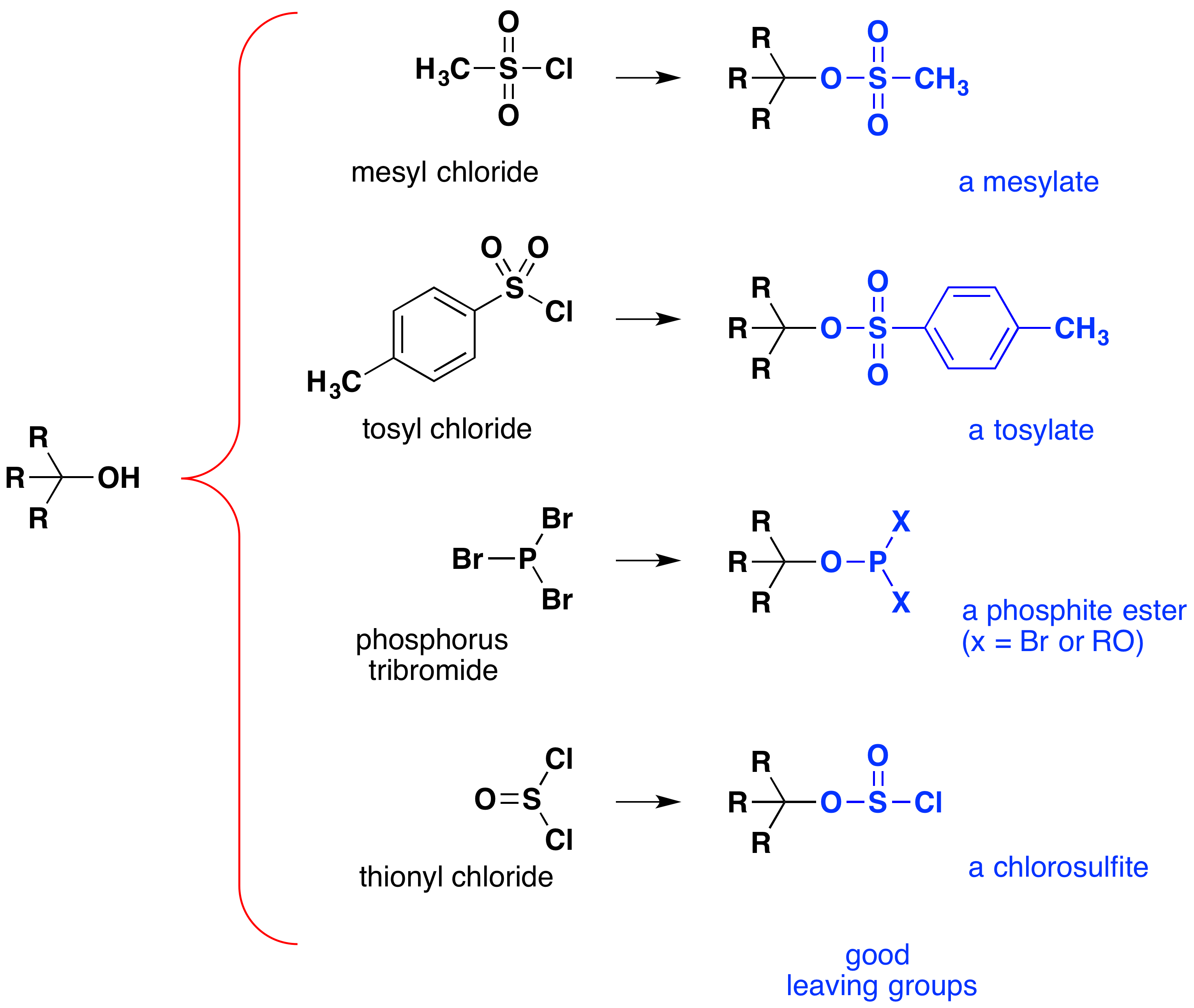
Although these reactions are sometimes referred to as “acid-catalyzed,” this is not strictly correct. In the overall transformation, a strong HX acid is converted to water, a very weak acid, so at least a stoichiometric quantity of HX is required for a complete conversion of alcohol to alkyl halide. The necessity of using equivalent quantities of very strong acids in this reaction limits its usefulness to simple alcohols of the type shown above. Alcohols with acid-sensitive groups do not, of course, tolerate such treatment. Nevertheless, the idea of modifying the -OH functional group to improve its stability as a leaving anion can be pursued in other directions. The following diagram shows some modifications that have proven effective. In each case the hydroxyl group is converted to an ester of a strong acid. The first two examples show the sulfonate esters described earlier. The third and fourth examples show the formation of a phosphite ester (X represents the remaining bromines or additional alcohol substituents) and a chlorosulfite ester, respectively. All of these leaving groups (colored blue) have conjugate acids that are much stronger than water (by 13 to 16 powers of ten); thus, the leaving anion is correspondingly more stable than the hydroxide ion. The mesylate and tosylate compounds are particularly useful because they may be used in substitution reactions with a wide variety of nucleophiles. The intermediates produced in reactions of alcohols with phosphorus tribromide and thionyl chloride (last two examples) are seldom isolated, and these reactions continue to produce alkyl bromide and chloride products.
The importance of sulfonate ester intermediates in general nucleophilic substitution reactions of alcohols may be illustrated by the following conversion of 1-butanol to pentanenitrile (butyl cyanide), a reaction that does not occur with the alcohol alone. The phosphorus and thionyl halides, on the other hand, only act to convert alcohols to the corresponding alkyl halides.
CH3CH2CH2CH2–OH + CH3SO2Cl
pyridine

CH3CH2CH2CH2–OSO2CH3
Na(+) CN(–)

CH3CH2CH2CH2–CN + CH3SO2O(–) Na(+)
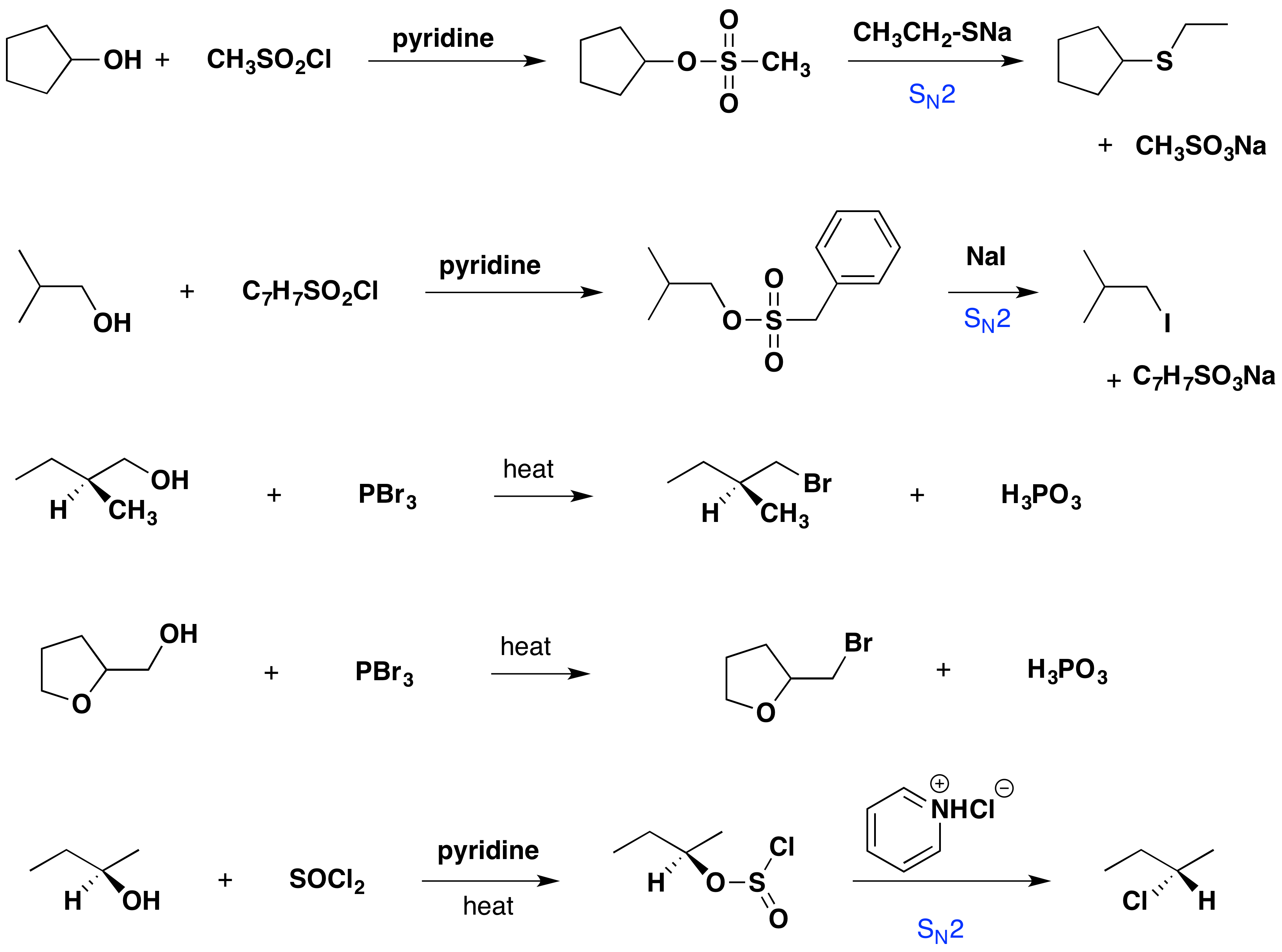
Some examples of alcohol substitution reactions using this approach to activating the hydroxyl group are shown in the following diagram. The first two cases serve to reinforce the fact that sulfonate ester derivatives of alcohols may replace alkyl halides in a variety of SN2 reactions. The next two cases demonstrate the use of phosphorus tribromide in converting alcohols to bromides. This reagent may be used without added base (e.g. pyridine) because the phosphorous acid product is a weaker acid than HBr. Phosphorus tribromide is best used with 1º-alcohols because 2º-alcohols often yield rearrangement by-products resulting from competing SN1 reactions. Note that the ether oxygen in reaction 4 is not affected by this reagent, whereas the alternative synthesis using concentrated HBr cleaves ethers. Phosphorus trichloride (PCl3) converts alcohols to alkyl chlorides in a similar manner, but thionyl chloride is usually preferred for this transformation because the inorganic products are gases (SO2 & HCl). Phosphorus triiodide is not stable but may be generated in situ from a mixture of red phosphorus and iodine and acts to convert alcohols to alkyl iodides. The last example shows the reaction of thionyl chloride with a chiral 2º-alcohol. The presence of an organic base such as pyridine is important because it provides a substantial concentration of chloride ion required for the final SN2 reaction of the chlorosufite intermediate. In the absence of a base, chlorosufites decompose upon heating to yield the expected alkyl chloride with retention of configuration
Tertiary alcohols are not commonly used for substitution reactions of the type discussed here because SN1 and E1 reaction paths are dominant and are difficult to control.
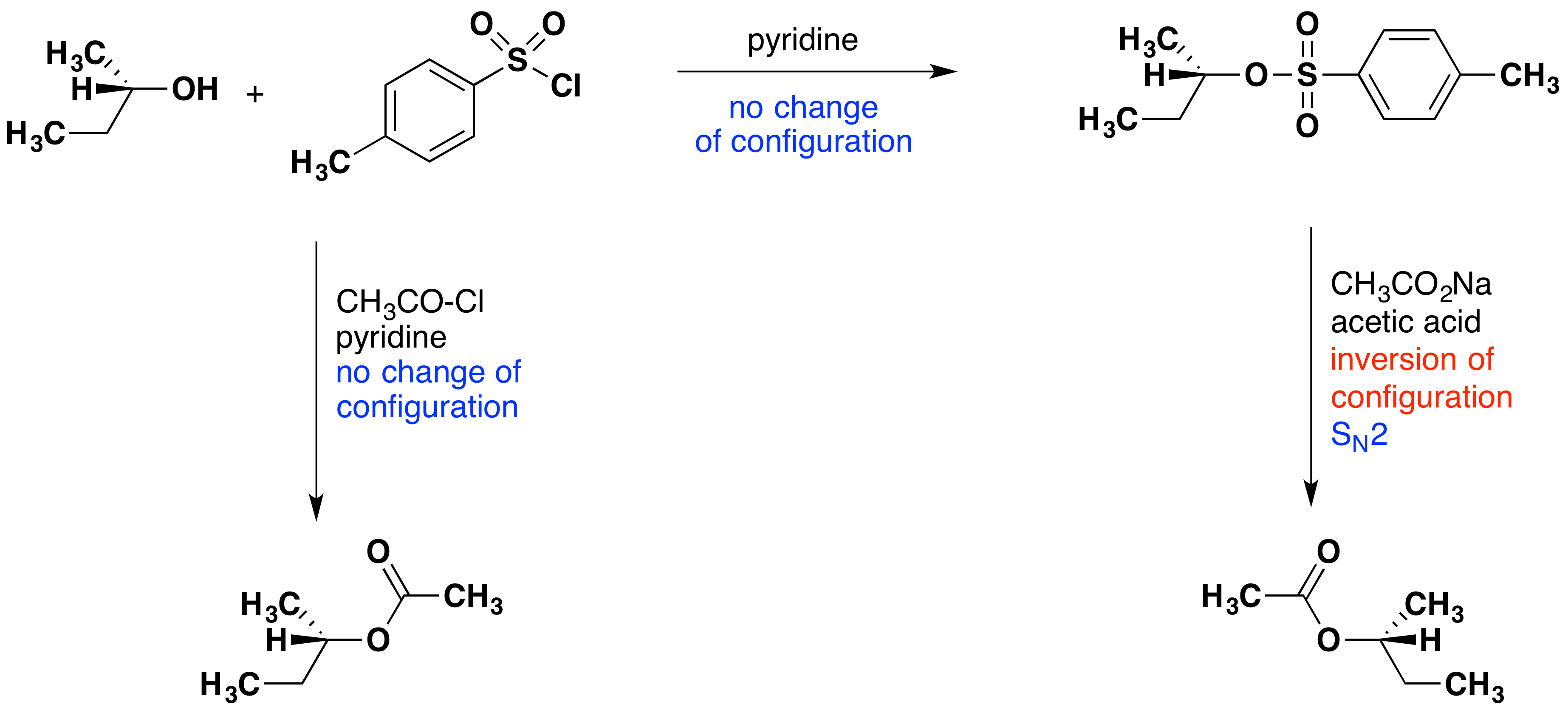
The importance of sulfonate esters as intermediates in many substitution reactions cannot be overstated. A rigorous proof of the configurational inversion that occurs at the substitution site in SN2 reactions makes use of such reactions. An example of such a proof is displayed below. Abbreviations for the more commonly used sulfonyl derivatives are given in the following table.
Sulfonyl Group
CH3SO2–
CH3C6H4SO2–
BrC6H4SO2–
CF3SO2–
Name & Abbrev.
Mesyl or Ms
Tosyl or Ts
Brosyl or Bs
Trifyl or Tf
Inversion Proof
For a more complete discussion of hydroxyl substitution reactions and a description of other selective methods for this transformation, Click Here.
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry






