Hydroxyl Group – Organic Chemistry Video
A hydroxyl group is an —OH group attached to another molecule. It can be found in alcohols, carboxylic acids, carbohydrates, and many more places.
What does the word hydroxyl mean?
Hydroxyl is not to be confused with hydroxide! Let’s break apart the pieces. Hydrox indicates that there are oxygen and hydrogen atoms, and the suffix -yl indicates that it’s a substituent of another molecule or that it’s missing a hydrogen and has no charge. That means that the hydroxyl group is attached to a parent molecule. The suffix -ide indicates a negative charge. Hydroxide is an anion, not a substituent on a parent molecule!
Where can the hydroxyl group be found?
The —OH group (hydroxyl group) appears in many types of molecules in chemistry, and the most common places to find it in O-Chem are alcohols, carboxylic acids, sulfonic acids, and carbohydrates. Let’s try to find them in these molecules:
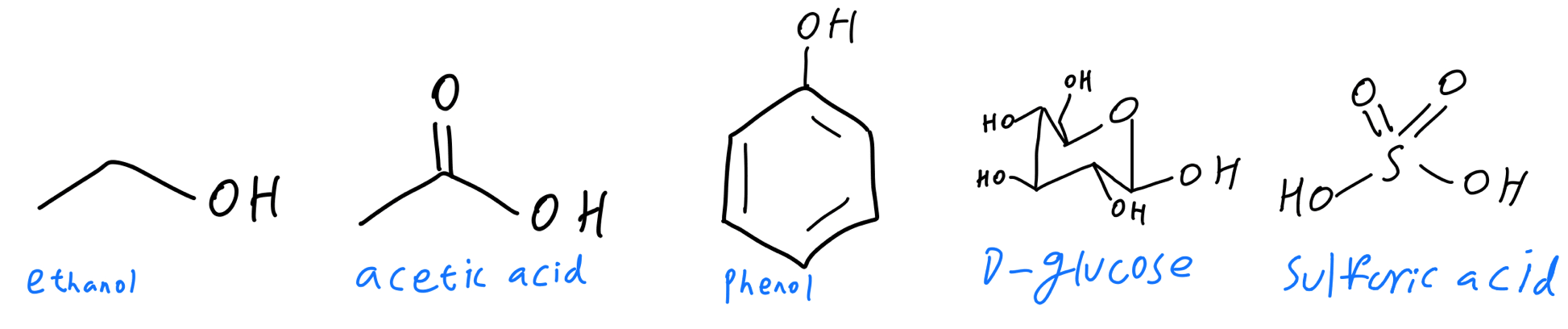 Unlabeled hydroxyl groups
Unlabeled hydroxyl groups
They were pretty easy to find, right? I’ve highlighted each hydroxyl group in each molecule below:
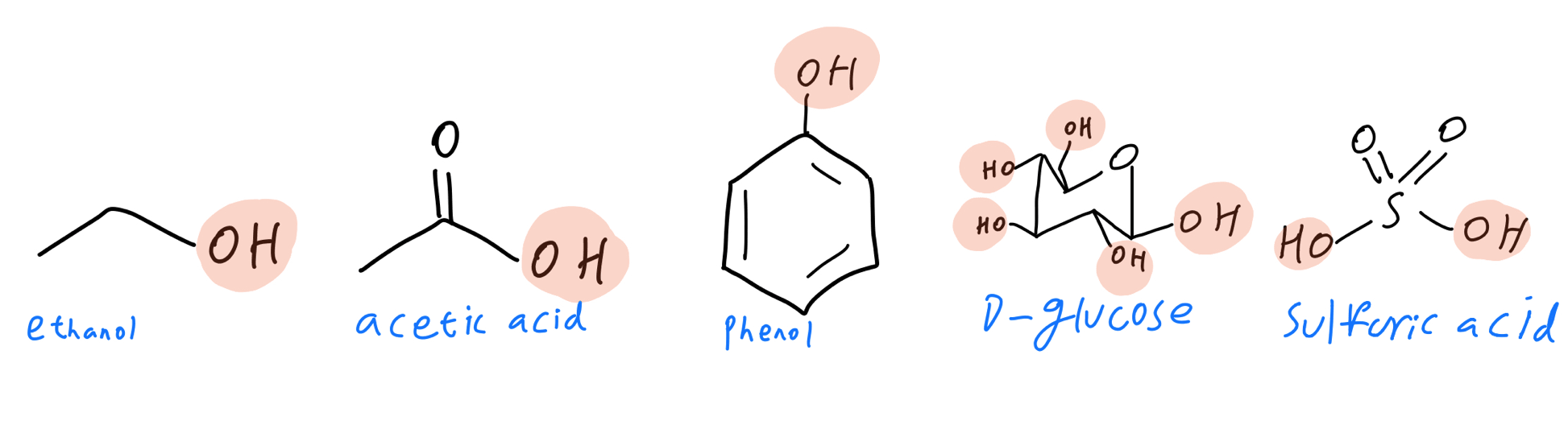
Properties:
Because oxygen is electronegative, many molecules with hydroxyl groups on them are actually soluble in polar solvents like water and are insoluble in nonpolar solvents like oils! But what about acidity? Hydroxyl groups, just like water, can function as either acids or bases. How it will act depends on a few factors including what functional group the hydroxyl group is part of as well as the concentration of protons in solution.
These are by no means the only molecules on which you can find a hydroxyl group, but it should impart the right idea. Want to learn about how to identify functional groups, determine solubility, or predict boiling point? Check out my videos on those topics here!






