What is the difference between amino groups and amine?
Complete answer:
Let us first understand what an amine is.
A compound that contains the basic nitrogen atom along with the lone pair is known as an amine.
Amines are usually a derivative of ammonia ($N{{H}_{3}}$).
Organic amines are formed when an alkyl or an aryl group replaces one or more hydrogen atoms from ammonia.
Monochloramine ($NCl{{H}_{2}}$) is an example of amine derived from inorganic derivatives of ammonia.
Now, a compound that contains the $-N{{H}_{2}}$ substituent group or moiety is known as the amino group. It is usually present in primary ($1{}^\circ $) amines.
So, the difference between amine and amino group is essentially a matter of nomenclature i.e., a compound containing basic nitrogen atom along with the lone pair is called an amine, and the functional group present in primary ($1{}^\circ $) amines is the amino group.
For example, $C{{H}_{3}}N{{H}_{2}}$ is an amine named methylamine and contains the amino functional group.
Note:
It should be noted that there are three types of amines depending upon the number of hydrogen atoms replaced by substituent alkyl or aryl groups in ammonia.
– Primary amine ($1{}^\circ $): 1 hydrogen atom is replaced by substituent alkyl or aryl groups in ammonia.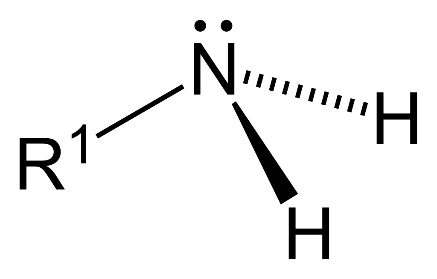
– Secondary amine ($2{}^\circ $): 2 hydrogen atoms are replaced by substituent alkyl or aryl groups in ammonia.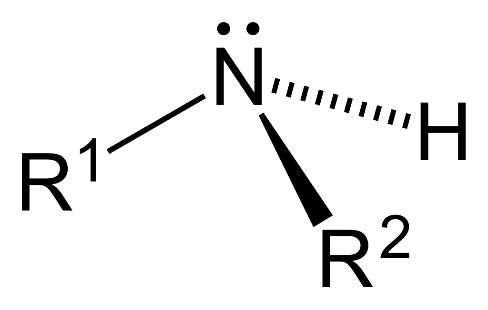
– Tertiary amine ($3{}^\circ $): 3 hydrogen atoms are replaced by substituent alkyl or aryl groups in ammonia.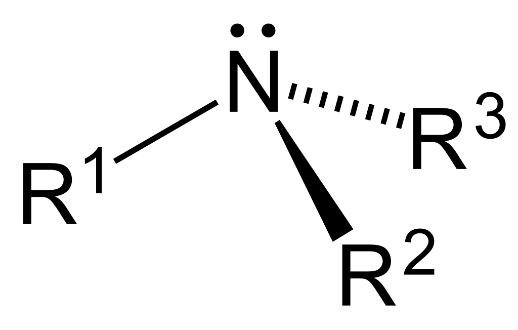
This question is based on the nomenclature of the functional group attached to an organic molecule. A functional group can be defined as the moiety or substance that affects and changes the characteristics of the chemical reaction of a molecule.Let us first understand what an amine is.A compound that contains the basic nitrogen atom along with the lone pair is known as an amine.Amines are usually a derivative of ammonia ($N{{H}_{3}}$).Organic amines are formed when an alkyl or an aryl group replaces one or more hydrogen atoms from ammonia.Monochloramine ($NCl{{H}_{2}}$) is an example of amine derived from inorganic derivatives of ammonia.Now, a compound that contains the $-N{{H}_{2}}$ substituent group or moiety is known as the amino group. It is usually present in primary ($1{}^\circ $) amines.So, the difference between amine and amino group is essentially a matter of nomenclature i.e., a compound containing basic nitrogen atom along with the lone pair is called an amine, and the functional group present in primary ($1{}^\circ $) amines is the amino group.For example, $C{{H}_{3}}N{{H}_{2}}$ is an amine named methylamine and contains the amino functional group.It should be noted that there are three types of amines depending upon the number of hydrogen atoms replaced by substituent alkyl or aryl groups in ammonia.- Primary amine ($1{}^\circ $): 1 hydrogen atom is replaced by substituent alkyl or aryl groups in ammonia.- Secondary amine ($2{}^\circ $): 2 hydrogen atoms are replaced by substituent alkyl or aryl groups in ammonia.- Tertiary amine ($3{}^\circ $): 3 hydrogen atoms are replaced by substituent alkyl or aryl groups in ammonia.






